Wow, you came off the blocks like a rocket!
Since you came off the blocks like a rocket in PDQ 1 and 2, are you ready for a bigger bite? Next stop, the Experiment!
PDQ2 |
Grades:
Time:
Subject:
5-8
5-15 minutes
Chemistry
Kaboom – it’s the 4th of July! Whether you’re watching fabulous fireworks or lighting a candle you’re watching a chemical reaction take place. This PDQ will fire up your scientific skills and introduce you to the science of burning candles. Let’s explore!
Chemical reactions take place around us all the time whether it is a car’s engine burning gasoline, your stomach digesting, or someone cooking dinner. The magic of chemistry creates new substances that didn’t exist when these reactions take place. Sometimes it’s a good thing and sometimes not so good if we think about pollution from internal combustion engines.
How all of this happens takes place at a microscopic level that we can’t see. All matter is made up of tiny particles called atoms and molecules and, at an even smaller level, atoms and molecules are made up of particles also! These sub-atomic particles – protons, neutrons, and electrons – have the ability to travel and exchange in certain circumstances and when they do, like magic, you have a new substance!

In this PDQ databot™ enters an airtight chamber with a burning candle to explore what’s going on in this unique chemical reaction. Every time you light a match or burn a candle you are engaging with a chemical reaction that creates new substances! The following diagram shows what a burning candle chemical reaction looks like. The paraffin wax of a typical candle is known as a hydrocarbon – it has a long chain of carbon atoms and hydrogen atoms are connected to it. The particles transform into new substances when fire – “combustion” -takes place!

Look closely at the equation and at the products of the reaction. Did you know that water (H2O) is being created when you burn a candle? By placing databot™ in an airtight chamber with a burning candle we will be able to contain these products and confirm this reaction using scientific sensors!
Ready to get started? Let’s fire this activity up and get going!
By completing this experiment and conducting the scientific observations associated with it you will master the following knowledge! Good luck science explorer!
Atom: The basic unit of matter and is the smallest thing that can have a chemical property. Hydrogen and Carbon are both examples of atoms.
Carbon Dioxide (CO2): A colorless, odorless gas naturally present in the air you breathe and is absorbed by plants in photosynthesis. There would be no animal life or green plants without carbon dioxide. Green plants use energy from the sun plus carbon dioxide and water to produce carbohydrates and oxygen. CO2 is one of the products of the vinegar-baking soda reaction comprised of 1 carbon atom and 2 oxygen atoms.
Chemical Reaction: When substances combine to produce one or more new substances.
Endothermic Reaction: A type of chemical reaction that requires energy to take place. When this happens you will see a drop in temperature in your reactants.
Exothermic Reaction: A type of chemical reaction that produces energy. When this happens you will see an increase in temperature.
Humidity: The percentage of water vapor in the air you breathe. You will notice humidity changes when you travel – for example, in the desert there is very low humidity and by the sea, you will have high levels of humidity.
Molecule: Made up of atoms and is the smallest amount of a chemical substance that still retains all the characteristics of that substance. A good example of a molecule is water. Represented as H2O, it has 2 hydrogen atoms and one oxygen atom.
Physical Reactions: When a substance changes form, but not its composition. For example, water turning from liquid form to ice. It’s still water, just in a different state.
Product: The substance created in a chemical reaction.
Reactant: The starting substance that enters into a chemical reaction.
States of Matter: The 4 states a substance can take on: solid, liquid, gas, or plasma.
Temperature: A measure of a physical property of a substance – how hot or cold is it? Temperature is actually a measure of moving particles in a substance and is expressed in different units such as degrees Celsius.
Units of Measure (metric): Liters, milliliters, parts per million (PPM).
Volume: The amount of space a substance takes up.
Water (H20): A product of the vinegar-baking soda reaction comprised of 2 hydrogen atoms and one oxygen atom. It is also a product of the chemical reaction that takes place when you burn a candle.
Now breathe on databot™and hold the temperature probe tightly to collect data and make sure everything is working properly! It should display CO2 and water vapor in your exhaled breath and the temperature should increase from your body heat.
Step 1: Let’s setup!

Step 2: Time to experiment!
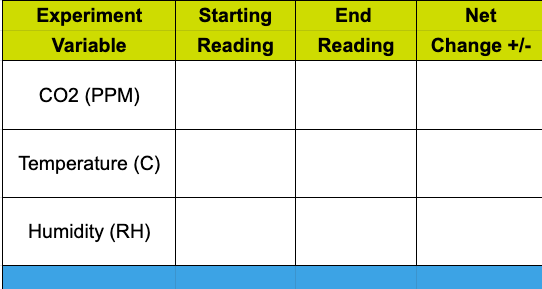
Observations and Deep Thoughts!
Calculate the net change recorded during the experiment for each variable.
Since you came off the blocks like a rocket in PDQ 1 and 2, are you ready for a bigger bite? Next stop, the Experiment!
Educator Info
This PDQ is a very simple activity to set up and perform. Conduct the experiment several times yourself to familiarize yourself with doing it smoothly and quickly.
Now breathe on databot™ and hold the temperature probe tightly to collect data and make sure everything is working properly! It should display CO2 and water vapor in your exhaled breath and the temperature should increase from your body heat.
This activity can be adjusted based on age groups and your desired experience. For younger students, you may simply want to introduce the concepts of chemical reactions and demonstrate an exothermic reaction. Older students have many opportunities to go much deeper with additional connections. This activity is extended in this module’s Challenge as well which provides additional opportunities for deeper learning.
This article is a deep treatment of student misconceptions related to fire.
Exploring Secondary Students’ Conceptions about Fire Using a Two-Tier,
True/False, Easy-to-Use Diagnostic Test
Patrice Potvin, Yannick Skelling-Desmeules, Ousmane Sy
Université du Québec à
A few examples from this paper:
David Rudel’s Science Myths Unmasked is an excellent source of additional information: the burning candle does not use up all of the oxygen in the jar, only about 30% of it.
Awesome and simple to follow information on the science of candles from the National Candle Association.
Highly recommended reading is Chapter 2 from David Rudel’s Science Myths Unmasked which addresses misconceptions about the candle in a jar experiment.
Incredible Caffeine Molecule image by Michael Ströck. Thank you!!
Candle image by Michael Schwarzenberger on Pixabay. Awesome!
 Ready, Set, Reaction! by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.
Ready, Set, Reaction! by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.