Great Work!
Great work! Another Chemical Reaction PDQ coming up! Ready. Set. Go!
PDQ1 |
Grades:
Time:
Subject:
5-8
5-15 minutes
Chemistry
Get ready to stir up some reaction action as we create a base + acid reaction using baking soda and vinegar and monitor for temperature and CO2 using databot™!
Mixing baking soda and vinegar is an exciting demonstration of a chemical reaction that is simple to whip up, but filled with interesting science to study. In this PDQ databot™ enters an airtight chamber to monitor CO2 levels and temperature to learn more about what is going on in this fabulous, fizzy mixture. As you prepare for this exciting exploration, check out the important terms below as well as the pH scale. Pay close attention to the terms endothermic and exothermic relating to temperature changes in the reaction.
Identify the reactants in the experiment as well as the products. Remember that a chemical change actually transforms a substance or substances into something new – a new substance! The following image provides an overview of the reaction.
Take a close look!

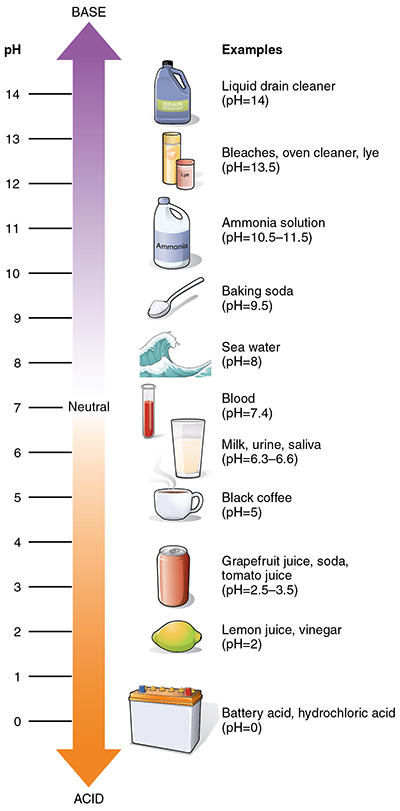
Can you find the reactants on the pH scale?
Ready to get started?
Prepare your databot™ with a full charge, test your connection to Phyphox, and let’s explore further with databot™!
By completing this experiment and conducting the scientific observations associated with it you will master the following knowledge! Good luck science explorer!
A set of inexpensive glass beakers can be very handy for the experiment.
Acid: A substance that has a pH level of less than 7, can donate a hydrogen ion, and is characterized by a sour taste or smell. If you have tasted pickle juice or smelled vinegar – those are both great examples of acids.
Baking Soda, sodium bicarbonate (NaHCO₃): An alkaline salt and is one of the reactants in the vinegar-baking soda reaction.
Base: A substance that has a pH level of higher than 7 and can accept a donated hydrogen ion from an acid. A base is essentially an acid’s opposite so when they get together some spectacular things can happen. Sodium bicarbonate (baking soda) is a good example of a base.
Carbon Dioxide (CO2): A colorless, odorless gas naturally present in the air you breathe and is absorbed by plants in photosynthesis. There would be no animal life or green plants without carbon dioxide. Green plants use energy from the sun plus carbon dioxide and water to produce carbohydrates and oxygen. CO2 is one of the products of the vinegar-baking soda reaction comprised of 1 carbon atom and 2 oxygen atoms.
Chemical Reaction: When substances combine to produce one or more new substances.
Endothermic Reaction: A type of chemical reaction that requires energy to take place. When this happens you will see a drop in temperature in your reactants.
Exothermic Reaction: A type of chemical reaction that produces energy. When this happens you will see an increase in temperature.
pH: A scale from 1-14 used to rate substances as either “acid” or “base” – the lower the number, the more acidic the substance. Pure water has a pH of 7.
Physical Reactions: When a substance changes form, but not its composition. For example, water turning from liquid form to ice. It’s still water, just in a different state.
Product: The substance created in a chemical reaction.
Reactant: The starting substance that enters into a chemical reaction.
Sodium Acetate (C2H3NaO2): One of the products of the vinegar-baking soda reaction comprised of carbon, hydrogen, oxygen, and sodium.
Temperature: A measure of a physical property of a substance – how hot or cold is it? Temperature is actually a measure of moving particles in a substance and is expressed in different units such as degrees Celsius.
Units of Measure (metric): Liters, milliliters, parts per million (PPM).
Vinegar: A mixture of acetic acid (CH₃COOH) and water (H20) that is one of the reactants in the baking-soda vinegar reaction.
Volume: The amount of space a substance takes up.
Water (H20): A product of the vinegar-baking soda reaction comprised of 2 hydrogen atoms and one oxygen atom.
Do a quick test by connecting to databot™ and starting the data recording. Now breathe on databot™and hold the temperature probe tightly to collect data and make sure everything is working properly! It should display CO2 in your exhaled breath and the temperature should increase from your body heat.
Step 1: Set up your “experiment chamber” as shown here. You need an airtight container that has a known volume that will capture the CO2 gas produced by the reaction.
Materials:

*Test your reactants in your glassware before doing the experiment in the chamber.
Step 2: Time to experiment!
Observations and Deep Thoughts!
Write down your data and findings in a format similar to the table on the right.

Great work! Another Chemical Reaction PDQ coming up! Ready. Set. Go!
Educator Info
By completing this experiment and conducting the scientific observations associated with it you will master the following knowledge! Good luck science explorer!
The following questions may or may not be appropriate for the age group you are working with so use your discretion.
https://www.stemmayhemHow should the reaction between vinegar and baking soda be classified?
https://antoine.frostburg.edu/chem/senese/101/reactions/faq/classify-vinegar-bakingsoda.shtml
Misconceptions about science
http://modeling.asu.edu/modeling/KindVanessaBarkerchem.pdf
 Ready, Set, Reaction! by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.
Ready, Set, Reaction! by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.