You’ve mastered some great challenges in this module.
Great job! Now for a new kind of adventure, prepare to be challenged to demonstrate your new-found knowledge and skills. Good luck!
Experiment |
Grades:
Time:
Subject:
5-8
20-30 minutes
Chemistry
Stirring up more reaction action as we further investigate the base + acid reaction discovered in PDQ 1. Prepare to don your lab coat and do some serious science. Let’s go!
Welcome to more endothermic explorations! Quiz time – what does the X-51A Waverider experimental US Air Force hypersonic aircraft have in common with a frying egg? Both are reliant on endothermic reactions! The reason your egg “cooks” is because it is absorbing heat energy from the pan which causes a chemical reaction. The X-51A flies at speeds in excess of 3,000 miles per hour which generates enormous heat. Engineers and scientists developed a special hyrdrocarbon fuel that actually absorbs heat and helps to cool systems in the aircraft so it can achieve these extraordinary speeds.

The amazing X-51A uses a special endothermic fuel to stay cool!

A frying egg is great example of an endothermic reaction in action!
In this experiment we go further and change things up by changing the ratio of the reactants to get more and bigger fizz!
Grab your databot™, and let’s find out!
By completing this experiment and conducting the scientific observations associated with it you will master the following knowledge! Good luck science explorer!
The glassware used in our demonstration to contain the reaction is a 250 ml beaker and this is adequate for the reaction. Study the video to better understand what you will need.
Acid: A substance that has a pH level of less than 7, can donate a hydrogen ion, and is characterized by a sour taste or smell. If you have tasted pickle juice or smelled vinegar – those are both great examples of acids.
Atom: The basic unit of matter and is the smallest thing that can have a chemical property. Hydrogen and Carbon are both examples of atoms.
Baking Soda, sodium bicarbonate (NaHCO₃): An alkaline salt and is one of the reactants in the vinegar-baking soda reaction.
Base: A substance that has a pH level of higher than 7 and can accept a donated hydrogen ion from an acid. A base is essentially an acid‘s opposite so when they get together some spectacular things can happen. Sodium bicarbonate (baking soda) is a good example of a base.
Carbon Dioxide (CO2): A colorless, odorless gas naturally present in the air you breathe and is absorbed by plants in photosynthesis. There would be no animal life or green plants without carbon dioxide. Green plants use energy from the sun plus carbon dioxide and water to produce carbohydrates and oxygen. CO2 is one of the products of the vinegar-baking soda reaction comprised of 1 carbon atom and 2 oxygen atoms.
Chemical Reaction: When substances combine to produce one or more new substances.
Density: An object’s mass in a given volume. For example, a 1 cm cube of gold is much denser than a 1 cm cube of balsa wood so the weight of the gold cube is much, much heavier.
Endothermic Reaction: A type of chemical reaction that requires energy to take place. When this happens you will see a drop in temperature in your reactants.
Exothermic Reaction: A type of chemical reaction that produces energy. When this happens you will see an increase in temperature.
Mass: The amount of matter in a substance.
Molecule: Made up of atoms and is the smallest amount of a chemical substance that still retains all the characteristics of that substance. A good example of a molecule is water. Represented as H2O, it has 2 hydrogen atoms and one oxygen atom.
pH: A scale from 1-14 used to rate substances as either “acid” or “base” – the lower the number, the more acidic the substance. Pure water has a pH of 7.
Physical Reactions: When a substance changes form, but not its composition. For example, water turning from liquid form to ice. It’s still water, just in a different state.
Product: The substance created in a chemical reaction.
Percentage: A proportion of one number compared to another and is based on a scale of 100. If you have 30 white rocks in a pile of 100 pebbles, you have 30% white rocks!
Ratio: A numeric comparison of one item to another. For example, if you have 3 white rocks and 2 black rocks, the ratio is 3 to 2 and is written as 3:2.
Reactant: The starting substance that enters into a chemical reaction.
Sodium Acetate (C2H3NaO2): One of the products of the vinegar-baking soda reaction comprised of carbon, hydrogen, oxygen, and sodium.
States of Matter: The 4 states a substance can take on: solid, liquid, gas, or plasma.
Temperature: A measure of a physical property of a substance – how hot or cold is it? Temperature is actually a measure of moving particles in a substance and is expressed in different units such as degrees Celsius.
Units of Measure (metric): Liters, milliliters, parts per million (PPM).
Vinegar: a mixture of acetic acid (CH₃COOH) and water (H20) that is one of the reactants in the baking-soda vinegar reaction.
Volume: The amount of space a substance takes up.
Water (H20): A product of the vinegar–baking soda reaction comprised of 2 hydrogen atoms and one oxygen atom. It is also a product of the chemical reaction that takes place when you burn a candle.
Weight: A measurement of the force of gravity applied to an object – it is calculated by multiplying an object’s mass by the acceleration of gravity. The weight of an object can vary depending on the gravitational field it is in.
Step 1: Set up your “experiment chamber” as you did for PDQ1. You need an airtight container that has a known volume that will capture the CO2 gas produced by the reaction. Materials:

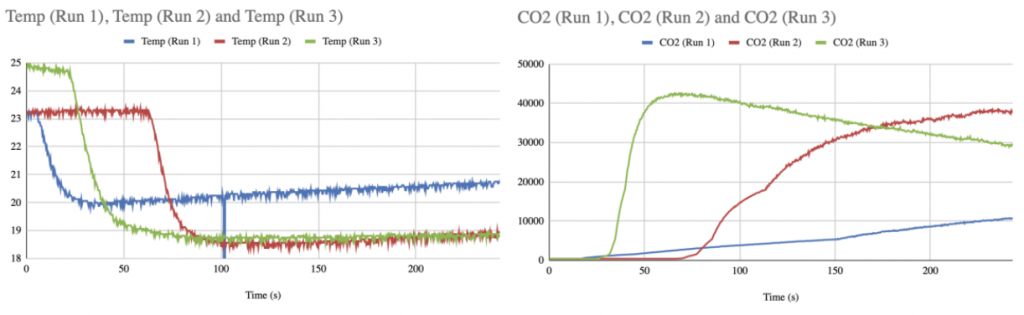
Step 2: Time to experiment! You will be doing this experiment three times, each time using a different ratio of reactants. Let’s go!
Reaction with a 6:1 ratio of reactants! Shown here is the reaction using 30 ml of vinegar (acetic acid) and 5 ml of baking soda (sodium bicarbonate). This is being conducted using a 250 ml beaker and we are getting close to overflow!! Make sure your glassware is the right size so you don’t overflow and soak databot™!
Things to observe!
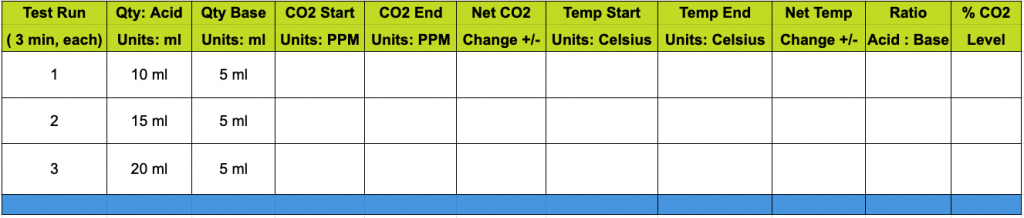
Complete this study by doing the math extensions using the worksheet. Note the two extra columns for ratios and percentages.
Great job! Now for a new kind of adventure, prepare to be challenged to demonstrate your new-found knowledge and skills. Good luck!
Educator Info
Understand and Recognize:
The following questions may or may not be appropriate for the age group you are working with so use your discretion.
How should the reaction between vinegar and baking soda be classified?
https://antoine.frostburg.edu/chem/senese/101/reactions/faq/classify-vinegar-bakingsoda.shtml
Misconceptions about science
http://modeling.asu.edu/modeling/KindVanessaBarkerchem.pdf
 Ready, Set, Reaction! by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.
Ready, Set, Reaction! by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.