Ready to get started? Let's go!
Next stop – PDQ1 – that means Pretty Darn Quick. Go dog, go!
Reactants, products, and fizzing fun all come together in this databot dive into the science behind chemical reactions!

Scan this QR Code with your Phyphox sensor app to load the CO2 sensor settings for all activities in the CO2 Science Series.
Reactants, products, and fizzing fun all come together in this databot™ dive into the science behind chemical reactions! Join databot™ as we stir up some chemical fun that provides insight into the invisible world of chemistry that surrounds us.
Grades:
Time:
Subject:
5-8
50 minutes (PDQ’s + Experiment)
50 Minutes (Challenge & Collaboration)
Chemistry
Poof – it seems like magic, but it’s not, it’s SCIENCE!! This engaging module introduces and explores the concept of chemical reactions – creating a new substance from two or more other substances.
The science of chemistry provides an exciting opportunity to explore the world around us and learn about the invisible forces that hold things together, cause them to come apart, create explosions, and burst your bubbles! Chemistry is literally the science of life and the materials that surround us.
Explore chemical reactions and see the difference between physical and chemical changes. Physical changes are when we see water freeze and it changes from a liquid to a solid. Or when we boil water and it changes from a liquid to a gas. The substance itself is not affected in a physical change – it’s still water, it’s just in a different “state of matter.”
When Chemical changes occur however the substance actually changes into something entirely new, such as our vinegar + baking soda reaction that changes these common materials into CO2, water, and sodium acetate.

In this module, the first PDQ kicks things off by watching for a temperature change and reading CO2 levels in a closed container. Temperature changes in chemical reactions are either endothermic (requiring energy) or exothermic (giving off energy). Using databot™ it’s fun and easy to monitor and visualize the changes that are taking place.
In the second PDQ, databot™ visualizes an exothermic reaction that results in the formation of water vapor and CO2. In each PDQ experimenting with changes in quantity or other variables produces visible changes in the data and suddenly it becomes apparent that we can change the output of our reaction!
The experiment following the PDQs poses the challenge to apply newfound skills to carefully control and produce the exact amount of CO2 desired in a closed environment. Be prepared to understand and master ratios and percentages as you attempt to create the perfect balance of CO2 in your own tiny micro-system.

By completing this experiment and conducting the scientific observations associated with it you will master the following knowledge! Good luck science explorer!
A set of inexpensive glass beakers can be very handy for the experiment
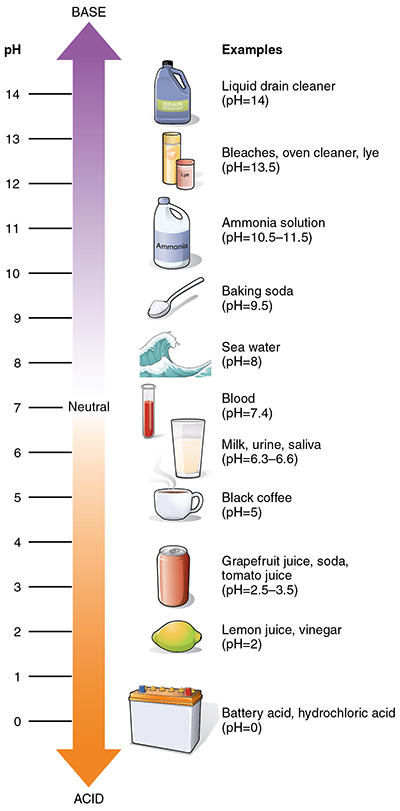
Acid: A substance that has a pH level of less than 7, can donate a hydrogen ion, and is characterized by a sour taste or smell. If you have tasted pickle juice or smelled vinegar – those are both great examples of acids.
Atom: The basic unit of matter and is the smallest thing that can have a chemical property. Hydrogen and Carbon are both examples of atoms.
Baking Soda: Sodium bicarbonate (NaHCO₃), is an alkaline salt and is one of the reactants in the vinegar–baking soda reaction.
Base: A substance that has a pH level of higher than 7 and can accept a donated hydrogen ion from an acid. A base is essentially an acid’s opposite so when they get together some spectacular things can happen. Sodium bicarbonate (baking soda) is a good example of a base.
Carbon Dioxide (CO2): A colorless, odorless gas naturally present in the air you breathe and is absorbed by plants in photosynthesis. There would be no animal life or green plants without carbon dioxide. Green plants use energy from the sun plus carbon dioxide and water to produce carbohydrates and oxygen. CO2 is one of the products of the vinegar-baking soda reaction comprised of 1 carbon atom and 2 oxygen atoms.
Chemical Reaction: This occurs when substances combine to produce one or more new substances.
Density: An object’s mass in a given volume. For example, a 1 cm cube of gold is much denser than a 1 cm cube of balsa wood so the weight of the gold cube is much, much heavier.
Endothermic Reaction: A type of chemical reaction that requires energy to take place. When this happens you will see a drop in temperature in your reactants.
Exothermic Reaction: A type of chemical reaction that produces energy. When this happens you will see an increase in temperature.
Humidity: The percentage of water vapor in the air you breathe. You will notice humidity changes when you travel – for example, in the desert there is very low humidity and by the sea, you will have high levels of humidity.
Mass: The amount of matter in a substance.
Molecule: Made up of atoms and is the smallest amount of a chemical substance that still retains all the characteristics of that substance. A good example of a molecule is water. Represented as H2O, it has 2 hydrogen atoms and one oxygen atom.
pH: A scale from 1-14 used to rate substances as either “acid” or “base” – the lower the number, the more acidic the substance. Pure water has a pH of 7.
Physical reaction: When a substance changes form, but not its composition. For example, water turning from liquid form to ice. It’s still water, just in a different state.
Product: The substance created in a chemical reaction.
Percentage: A proportion of one number compared to another and is based on a scale of 100. If you have 30 white rocks in a pile of 100 pebbles, you have 30% white rocks!
Ratio: A numeric comparison of one item to another. For example, if you have 3 white rocks and 2 black rocks, the ratio is 3 to 2 and is written as 3:2.
Reactant: The starting substance that enters into a chemical reaction.
Sodium Acetate (C2H3NaO2): One of the products of the vinegar–baking soda reaction comprised of carbon, hydrogen, oxygen, and sodium.
States of Matter: The 4 states a substance can take on: solid, liquid, gas, or plasma.
Temperature: A measure of a physical property of a substance – how hot or cold is it? Temperature is actually a measure of moving particles in a substance and is expressed in different units such as degrees Celsius.
Units of Measure (metric): Liters, milliliters, parts per million (PPM).
Vinegar: A mixture of acetic acid (CH₃COOH) and water (H20) that is one of the reactants in the baking-soda vinegar reaction.
Volume: The amount of space a substance takes up.
Water (H20): A product of the vinegar–baking soda reaction comprised of 2 hydrogen atoms and one oxygen atom. It is also a product of the chemical reaction that takes place when you burn a candle.
Weight: A measurement of the force of gravity applied to an object – it is calculated by multiplying an object’s mass by the acceleration of gravity. The weight of an object can vary depending on the gravitational field acting upon it.
Read the background information, study the terms, and explore the additional resource links.
Next stop – PDQ1 – that means Pretty Darn Quick. Go dog, go!
Educator Info
Understand and Recognize:
Images
Definitions and Sources
 Ready, Set, Reaction! by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.
Ready, Set, Reaction! by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.