Cool bananas – you are now a chemical engineer demonstrating mastery of your environment!
It’s now time for some community and group hugs. Next and final stop – a collaboration activity. Enjoy!
Challenge |
Grades:
Time:
Subject:
5-8
30 minutes or longer
Chemistry, Engineering
In PDQ 2 you recorded data as your candle burned. Now you are challenged to demonstrate your mastery over CO2 by carefully controlling it in a closed environment.
After studying chemical reactions and understanding the behavior of CO2 gas generated in the candle experiment, you are now challenged to go further! You will now add additional candles to your closed chamber experiment and come to a deeper understanding of the relationship between the exothermic reaction in the candles and the production of CO2 gas. Get ready for the ultimate challenge as you demonstrate your understanding of this reaction and use it to control exactly when your candles will extinguish.
By completing this experiment and conducting the scientific observations associated with it you will master the following knowledge! Good luck science explorer!
These are the same materials used PDQ 2 with the addition of extra candles and blocks to place the candles at different heights.
As a team or individually experiment with the timing of when your candle(s) extinguish inside your test chamber. There are several variables associated with this challenge:
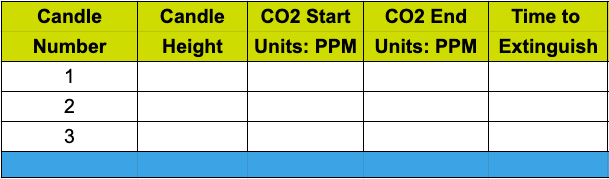
Use the following table to record your findings and take careful notes. Look for patterns!
Question: Why do candles at different heights go out at different times?
After experimenting with the different configurations, you are ready for the big challenge! Using all your scientific knowledge, now place the number of candles required at the correct heights to carefully control the CO2 production and put out the candles at precisely 95 seconds.
Please note that you may need to adjust your timing challenge based on the “chamber” size you are using. The experiments conducted in this module were all done in a 4 liter container. If you have a smaller or larger container you may need to adjust.
Go Further
Extend your mastery by trying some modifications!
Deep Thoughts to Ponder
If you conducted the Cave of Dogs activity, you observed the behavior of candles when in the presence of CO2. Does your experiment here match the behavior you saw in the Cave of Dogs? Why or why not?
It’s now time for some community and group hugs. Next and final stop – a collaboration activity. Enjoy!
Challenges like this truly encourage critical thinking and mastery by the students. By carefully gathering data and creating a final working solution students will better understand some of the variables going on inside the test chamber. By learning to control the output they are demonstrating a certain level of mastery.
Time permitting, the modifications suggested will provide further reinforcement of the overall module learning objectives.
One of the interesting things in this experiment is that the candles will go out from the highest to lowest. As the candles generate heat as well as CO2, the hot layer of air traps the CO2 above the candles, but as CO2 accumulates its density and increasing abundance push down from the top, extinguishing the candles one by one. In the Cave of Dogs experiment, we have an open container releasing the heat of the candles and feed the CO2 in from an alternate source. Consequently, its higher density causes the CO2 to settle lower in the chamber and build up, putting the candles out from the bottom up. For students who have done both activities, this is a great challenge for them to explain why this happening. Why do they go out bottom up in one experiment and top down in another? Science!
Educator Info
 Ready, Set, Reaction! by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.
Ready, Set, Reaction! by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.