The Cave of Dogs
PDQ1 |
Catch that gas!
Overview
Grades:
Time:
Subject:
Topics:
5-8
5-15 minutes
Chemistry
Properties of Gasses,
Chemical Reactions
Using simple tools and materials Carbon Dioxide (CO2) is generated
and captured in a spectacular example of a chemical reaction!
Once we know how to generate and capture CO2 we will put it to work
in our grand Cave of Dogs simulation.
Background
Mixing baking soda and vinegar is an exciting demonstration of a chemical reaction and a great way to see physical and chemical changes. Work with CO2 to learn how it behaves like a gas, in what ways it can be harmful, how to control it, and how to measure it. Ready for some chemical fun? Let’s explore further with databot™!
Objectives
Understand and Recognize:
- Different types of substances can “react” with one another to create a chemical change in the substances.
- Chemical reactions can cause physical and chemical changes in substances – even creating gas where there was none before.
- CO2 can be generated through a chemical reaction of baking soda and vinegar.
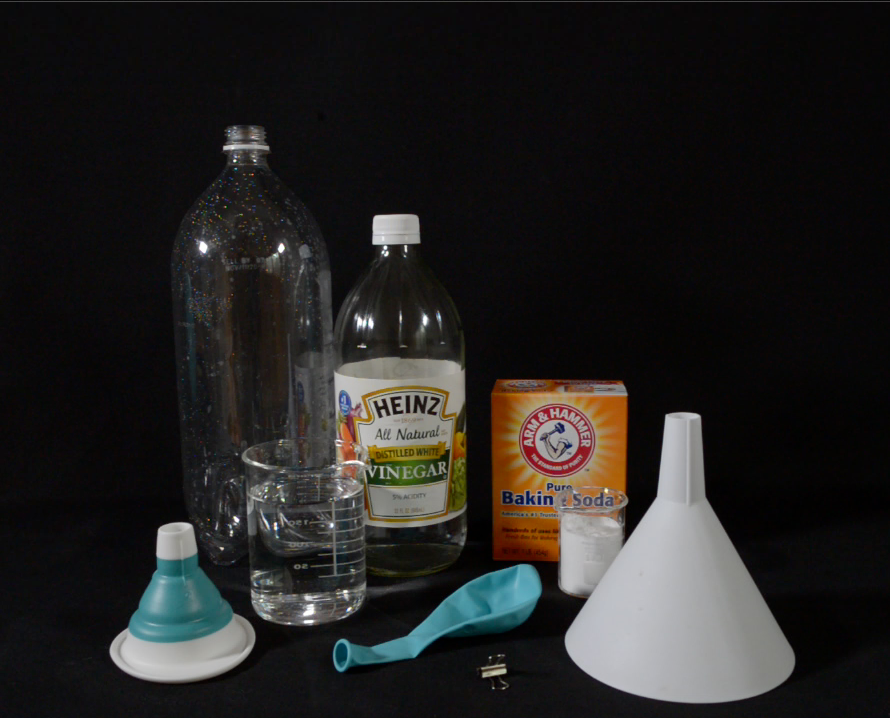
What You'll Need
- 2 Liter Plastic Bottle
- 12″ Round Balloons (3 or more recommended)
- White Vinegar (Acetic Acid)
- Baking Soda (Sodium Bicarbonate)
- Measuring Cups
- Small funnels to help control the pouring of the reactants.
- Small binder clip or paper clip to pinch off the balloon.
Important Terms
Carbon Dioxide (CO2): A colorless, odorless gas naturally present in the air you breathe and is absorbed by plants in photosynthesis. There would be no animal life or green plants without carbon dioxide. Green plants use energy from the sun plus carbon dioxide and water to produce carbohydrates and oxygen.
Volume: The amount of space a substance takes up.
Chemical Reaction: This occurs when substances combine to produce one or more new substances.
Reactant: The starting substance that enters into a chemical reaction.
Product: The substance created in a chemical reaction.
Prep (5 Mins)
- Organize your reactants and materials for quick and easy access.
- Be aware that you don’t want to accidentally combine your reactants before you are ready, so keep the baking soda (sodium bicarbonate) and vinegar (acetic acid) separate!
- Think about the order of your procedure in advance so you are prepared to execute smoothly!
- Be tidy and keep your workspace clean.
PDQ 1 (10 mins)
- Pour 1 cup of acetic acid (vinegar) into your 2 liter bottle using a funnel (Metric: use 250 ml of vinegar).
- Add 1/4 cup of sodium bicarbonate to your balloon (Metric: use 50 ml of baking soda). Use a separate funnel or carefully clean the one used for the vinegar to prevent an early reaction.
- Fix the balloon around the opening of your bottle.
- Carefully shake the sodium bicarbonate into the bottle and watch the reaction take place!
- Your balloon will fill with CO2. If you are planning on doing PDQ 2 in the same session, use your small binder clips from that activity to pinch off the balloon opening and preserve the CO2 for what’s coming next!
- Now that you know how to generate and capture CO2 in this fashion, additional experiments can be done!
What are the reactants in this experiment? What are the products? Do you see a chemical change or a physical change?
Educator Info
Educator Info
- Study the background information in the Overview and familiarize yourself with the learning objectives and terms for this activity.
- Set-up and test the CO2 experiment and conduct the PDQ yourself before conducting it for your class.
- Review the guiding questions to help guide the student experience.
- If students are conducting the experiment it’s recommended to start with the acetic acid first, then distributing and handling the sodium bicarbonate after to keep the reactants separate. Plan for disposal of the liquid product in a tidy fashion
Understand and Recognize:
- Different types of substances can “react” with one another to create a chemical change in the substances.
- Chemical reactions can cause physical and chemical changes in substances – even creating gas where there was none before.
- CO2 can be generated through a chemical reaction of baking soda and vinegar.
- 5-PS1-4: Conduct an investigation to determine whether the mixing of two or more substances results in new substances.
Gases such as air and CO2 are invisible and as such, pose an interesting challenge for students to develop understanding. Activities in the Cave of Dogs module provide clear demonstrations about gases such as air and CO2 that will help students form a deeper understanding of the characteristics of gases.
- Air is weightless and floats.
- All gases are weightless.
The following questions may or may not be appropriate for the age group you are working with so use your discretion.
- What are the states of matter?
- What states of matter do we see in our experiment here?
- What are the reactants in our experiment?
- What is the product?
- Is this a physical change or a chemical change?
- Does it appear we have created new substances in our experiment?
- How could we get our baking soda and vinegar back to normal?
- Do you suppose this experiment could be dangerous if we were careless? How?
How should the reaction between vinegar and baking soda be classified?
https://antoine.frostburg.edu/chem/senese/101/reactions/faq/classify-vinegar-bakingsoda.shtml
Misconceptions about science
http://modeling.asu.edu/modeling/KindVanessaBarkerchem.pdf
Why Does Vinegar and Baking Soda React?
https://www.stemmayhem.com/why-does-vinegar-baking-soda-react/
 The Cave of Dogs by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.
The Cave of Dogs by Robert O. Grover & Team databot™ is licensed under a Creative Commons Attribution 4.0 International License. Permissions beyond the scope of this license may be available at databot.us.com/contact.
